Introduction
Materials and Methods
Plants and soils
Preparation of thallium-contaminated soil
Growing procedure for plants in soil-culture
Determination of thallium in plants
Statistical analysis
Results and Discussion
Conclusions
Introduction
Thallium (Tl) is toxic to all organisms and it can be easily absorbed through skin and mucous membranes (Cheam, 2001). It is widely distributed in the environment. The concentration of Tl in surface soils ranged approximately from 0.1 to 2.0 mg kg-1, but most soils contain less than 1.0 mg kg-1 (Qi et al., 1992). In particular, some locations such as cement plants and old mines in China contained between 15 and 73 mg kg-1 (Zhou and Liu, 1985). The distribution of Tl in soils including agricultural fields near cement plants, abandoned mines and smelter areas in Korea was measured between 0.18 and 12.91 mg kg-1 (Lee et al., 2015). Specific guidelines for thallium in Korean soils has not been established yet (KMOE, 2021). However, Thallium contamination level in Canada is limited to 1.0 mg kg-1 in agricultural, residential, commercial, and industrial lands (CCME, 2007). A generic specific screening level for thallium in the USA is 6 mg kg-1 in residential areas (USEPA, 2002).
Thallium forms its oxides in two different oxidation states, monovalent thallous Tl(I), Tl+, and trivalent thallic Tl(III), Tl3+. In plants, most of the thallium found in plants is the uncomplexed ionic Tl+ species because Tl+ does not complex with other materials and is not transformed into Tl3+ (Mestek et al., 2007). Thus, the weakly reactive Tl+ is highly soluble and more bioavailable Tl species in soil and water environments (E&CC Canada, 2020).
Plant uptake of Tl+ is similar to the absorption of other monovalent ions, particularly the alkali cations such as K+, Cs+ and Rb+ because the Tl+ has a similar ionic radius and a similar low hydration enthalpy (Nriagu, 1998; Rao et al., 2008; Wick et al., 2018, 2020). Also, cucumber and bean plants has been generally used to evaluate uptake of heavy metals (Hardiman et al., 1984; Xian, 1989; Mansour et al., 2009; Jalali and Mojahed, 2020), and also these plants are widely cultivated as food crops in Korea. Many of food crops have been found to accumulate Tl at different concentrations including beetroot, chili pepper, eggplant, green bean, green cabbage, kale, lettuce, maize, onion, radish, spinach, tomato, watercress, and white mustard (Kurz et al., 1999; LaCoste et al., 2001; Pavlickova et al., 2006; Wang et al., 2013; D'Orazio et al., 2020).
In our environmental and agricultural systems, the applications of synthetic chelating agents such as ethylene diamine tetraacetic acid (EDTA), diethylene triamine pentaacetic acid (DTPA) and ethylene glycol tetraacetic acid (EGTA) markedly improved the phytoextraction of heavy metals including Tl by non-hyperaccumulator and hyperaccumulator plant species (Pereira et al., 2010; Seth et al., 2011; Souza et al., 2013). In contrast, these types of chelate agents, EDTA, DTPA, and Ethylenediamine-N,N'-disuccinic acid (EDDS), decrease or do not improve the uptake and translocation of copper (Cu), zinc (Zn), and lead (Pb) by various food crops (Yeh and Pan, 2012). Also, Tl concentrations in the barley tissues increased with DTPA applications, but Tl in the sunflower tissues decreased with DTPA treatments in hydroponic culture (Kim et al., 2016). However, Tl has been studied to a much lesser degree than other toxic trace metals or metalloids. With this reason, Tl bioaccumulation of plants, especially in food crops, in Korea has not been much considered.
Therefore, the aim of this study was to investigate the uptake and translocation of Tl in the selected food crops, Cucumis sativus and Phaseolus vulgaris, from artificially Tl-contaminated soils with or without the application of DTPA.
Materials and Methods
Plants and soils
Seeds of two plant species were obtained from different sources as follows; the seeds of cucumber (Cucumis sativus) were purchased from Monsanto Korea and the seeds of bean (Phaseolus vulgaris) were gained from the Seed Center of Jeollabuk-Do Agricultural Research & Extension Services, Korea.
Thallium (Tl)-free acidic (AJJ) and neutral (NIS) soils were collected from Jeonju and Iksan in Jeollabuk-Do, Korea, respectively. The collected soil samples were crushed to pass through a 2-mm sieve after air-dried and stored in plastic containers. The soil pH was measured using soil: solution ratios of 1:5 with a Professional pH Meter PP-20 (Sartorius®, Taylor Scientific). Soil organic matter content was determined by the Tyurin titrimetric method. Exchangeable cations were determined with the use of 1.0 M ammonium acetate (CH3COONH4, pH 7.0). The chemical properties of the soils were determined using the methods recommended by National Academy of Agricultural Science, RDA, Korea (NAAS, 2010). Total Tl concentration in the soils was determined by the pseudototal aqua regia (3 HCl:1 HNO3) digestion procedure (ISO 11466, 1995). Particle-size distribution was determined by a modified procedure based on the methods developed by Malo and Doolittle (2000). Selected chemical and physical properties of the soils were shown in Table 1.
Table 1
Selected physical and chemical properties of different soils studied.
Preparation of thallium-contaminated soil
A set of triplicate 300 g (air-dry basis) soils, acidic Jeonju and neutral Iksan soils, was placed into polyethylene zipper bags. The soils were treated with 0, 5, 10, 20 and 40 mg Tl kg-1 using TlCl solution. The soil contamination levels of Tl were decided based on the concentrations of Tl distribution in Korea (0.18 - 12.91 mg kg-1) and other countries (15 - 73 mg kg-1). Another set of the soils was also prepared with a fixed concentration of 10 mg Tl kg-1 combining with 0, 5, and 30 mg kg-1 of diethylene tetramine pentaacetic acid (C14H23N3O10, DTPA). The Tl contaminated soils with/without DTPA in zipper bags were thoroughly mixed by gently squeezing from outside of the bag and then incubated at 24 ± 2°C for 30 days. After incubating, the soils were transferred into a 500 mL polypropylene wide-mouth bottle pot in preparation for planting.
Growing procedure for plants in soil-culture
The plant seeds were soaked in deionized water for approximately 6.0 hours and then spread between two damp filter papers in plastic Petri dishes for 7 - 10 days. They were placed in a dark condition for overnight to germinate. Sprouted seeds were planted in experimental pots filled with seedbed material and provided with nutrient solution. The nutrient solution was prepared by the method reported from Mori and Nishizawa (1987). The seedlings were grown to approximately 10 - 15 cm in shoot height. Each seedling was carefully removed from the pots. The roots of plants were washed with tap water and then rinsed with deionized water to remove attached seedbed materials. The young plants were stabilized in the same nutrient solution for at least 3 hours and then transplanted into each labeled Tl-contaminated soil-culture pot. The proper volume of deionized water was applied to bring the soil to 80 - 90% of field capacity. The plant was grown for 7 days in environmental growth chambers with following conditions (Lee et al., 2002; Kim et al., 2016): temperatures were maintained at 20 ± 2°C for cucumber and at 25 ± 2°C for bean plant with a 12 h light cycle (82.5 - 95.8 µE m2 S-1) and 60 - 70% relative humidity. Water loss in the pots by evapotranspiration was replaced with a solution of 0.1% (w/w) of NH4NO3.
Determination of thallium in plants
After harvesting the plants, soil particles on the plant roots were completely removed with tap water and then the whole plants were rinsed with deionized water. Moisture on the plants was dried off with a laboratory paper towel. The plants were dried for 24 h at 80°C in a drying-oven after separated into shoot and root parts. The oven-dried plant tissues were digested in concentrated HNO3 and 30% H2O2 (Jones, 1991) to determine Tl concentration. Thallium concentration was measured using inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 7300DV, Perkin Elmer, USA). The selected Tl wavelength value was 190.801.
In addition, translocation index (TLI, %) for Tl in the plants was calculated as the ratio of the Tl concentration in the shoot of the plant (dry mass basis) to the Tl concentration in the whole plant: TLI (%) = [(TlST)/(TlST + TlRT)] × 100, where TlST and TlRT are the Tl concentrations in the shoot and root, respectively (Lee et al., 2002; Hladun et al., 2015; Kim et al., 2016). Bioaccumulation factor (BAF) for Tl was also estimated by BAF = TlST/TlSOIL, where the TlSOIL is Tl concentration in soil (Ma et al., 2001).
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) ver. 18.0 (SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test (DMRT) was carried out to check for the significance of Tl concentration in plants, bioaccumulation factor (BAF), and translocation index (TLI). Pearson’s correlation analysis was used to estimate the relations between thallium uptake and translocation in cucumber and bean plants grown in the artificially Tl contaminated soils.
Results and Discussion
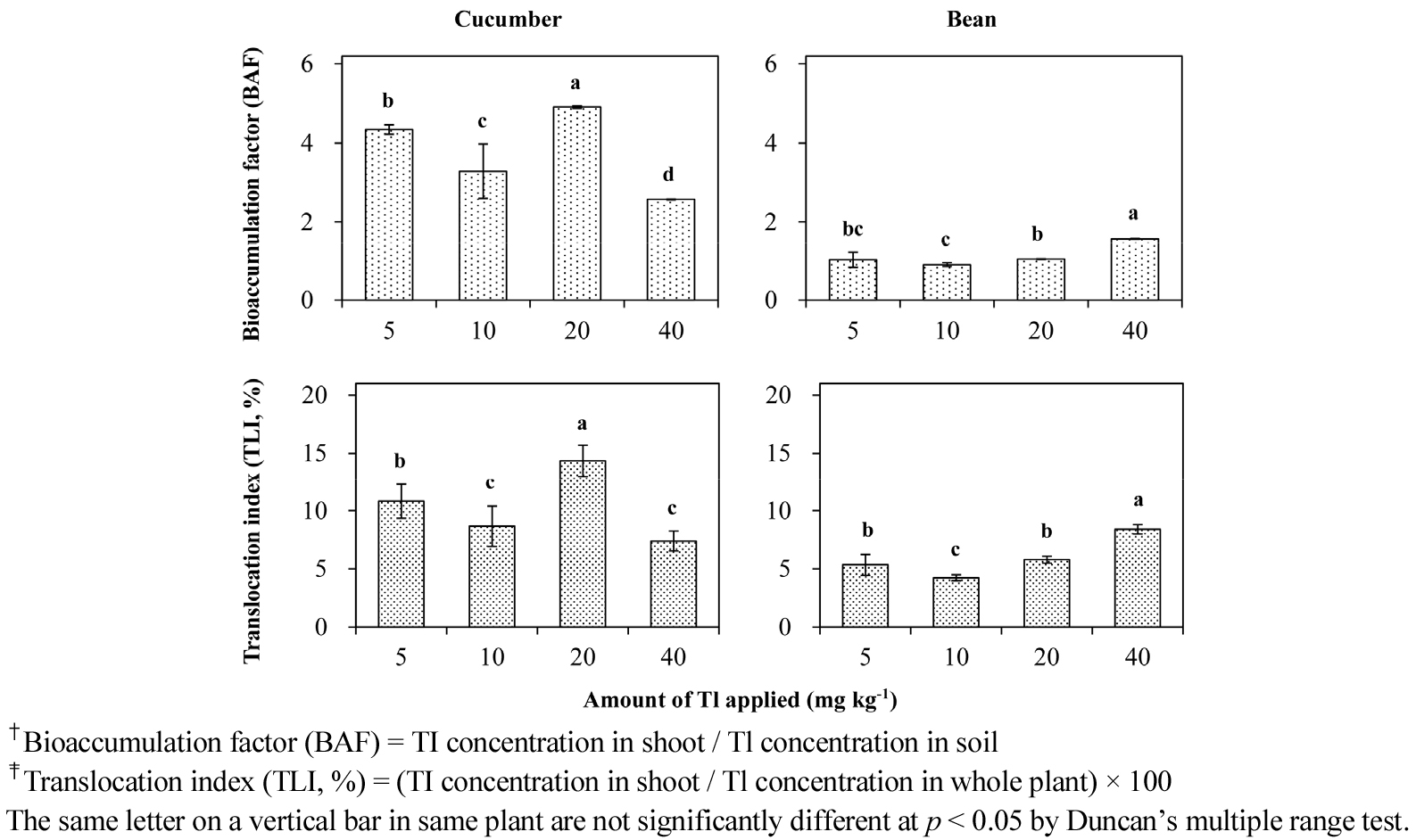
Uptake and translocation of Tl in cucumber and bean plants as affected by different Tl concentrations in acidic (AJJ) and neutral (NIS) soils are shown in Fig. 1. The concentrations of Tl in the shoots and roots of cucumber and bean plants increased with increasing Tl concentrations in the soils. The shoot Tl concentrations of plants in the AJJ soil ranged from a low of 5.2 mg Tl kg-1 for bean to a high of 102.7 mg Tl kg-1 for cucumber. Thallium in the shoots of plants in the NIS soil was distributed from a low of <0.1 mg Tl kg-1 for bean to a high of 21.5 mg Tl kg-1 for cucumber. The root Tl concentrations of plants in the acidic soil were between a low of 91.8 mg Tl kg-1 for bean and a high of 685.8 mg Tl kg-1 for cucumber. The Tl concentrations in the roots of plants in the NIS soil were from a low of 8.5 mg Tl kg-1 for bean to a high of 254.3 mg Tl kg-1 for cucumber. These results indicated that Tl uptake by cucumber plant was always higher than by bean plant. Besides, the root Tl concentrations of both the plants were much higher than the shoot Tl concentrations, which might come from the fixation of positively charged ions on root epidermal cells. Cell walls in root epidermis and apoplast have apparent free spaces (AFS) that are composed of water free spaces (WFS) and Donnan free spaces (DFS). The WFS are freely accessible to ions and molecules because they are macropores, whereas the DFS are micropores and have carboxylic groups that attract cations and play as cation exchangers. The number of exchange sites are dependent upon plant species (Vyas and Mistry, 1983; Marschner, 1995).
The Tl concentrations in the shoots and roots of plants were markedly higher in the acidic soil than in the neutral soil. Metal bioavailability in soils is typically changed by the adsorption/desorption reactions of metal ions in soil-water system, which is dependent upon soil properties including soil pH, organic matter, dissolved organic carbon, low-molecular weight organic acids, and index cations. In particular, decrease in soil pH produces increase in metal bioavailability through desorption of metal ions on protonated binding sites (Buchter et al., 1989; Kim et al., 2007).
Bioaccumulation factor (BAF) and translocation index (TLI) of Tl for cucumber and bean plants as influenced by different Tl concentrations in the AJJ and NIS soils were also presented in Fig. 1, respectively. Some of Tl-BAF values for cucumber and bean plants showed a specific trend with different Tl concentrations in both the soils. Only the BAF value of Tl for bean plant in the AJJ soil increased with increase in soil Tl concentration. Similar trends were observed with Tl-TLI values for the plants. Thallium TLI values for bean plant increased with increasing Tl concentrations of the soils. The TLI values for bean plant ranged from approximately 4.23% to 8.40% in the AJJ soil and from about lower than 1.2% to 4.82% in the NIS soil. Also, the Tl-TLI values for cucumber plant ranged from 7.39% to 14.36% in the AJJ soil and from 7.87% to 11.11% in the NIS soil. The BAF and TLI values of Tl were always higher for cucumber plant than for bean plant in both the acidic and neutral soils. Pettersson (1976) reported that manganese (Mn), nickel (Ni) and lead (Pb) exhibited the greatest mobility in cucumber plant as compared with rape, wheat, oats and tomato, and there was the highest ratio of shoot metal concentration to root metal concentration.
Correlation coefficient (r) relating Tl uptake and translocation in cucumber and bean plants as affected by different concentrations of Tl contaminated in the AJJ and NIS soils were shown in Table 2. The Tl concentrations in the shoots of cucumber and bean plants were significantly correlated (at p < 0.001) with those in the roots of the plants grown in the same soils. The correlation coefficient values ranged from 0.814 to 0.984 for the plant species. Also, the shoot or root Tl concentrations of cucumber and bean plants in the AJJ soil were significantly correlated (at p < 0.001) with Tl in the same parts of each plant in the NIS soil, which showed strong correlations with correlation coefficient values ranging from 0.879 to 0.971.
On the other hand, the shoot Tl concentrations of cucumber plant were not correlated with the Tl-BAF values for the plant in both the acidic and neutral soils, even though the Tl-BAF value depended on plant shoot Tl and soil Tl concentrations. These results indicated that Tl toxicity with the highest soil Tl concentration might affect Tl transport from roots to shoots of the plant. However, the shoot Tl in bean plant were significantly correlated (r = 0.806 to 0.913 at p < 0.001) with the Tl-BAF values for the plant in both the different soils.
Table 2
Correlation coefficient (r) relating thallium uptake and translocation in cucumber and bean plants grown in the artificially Tl contaminated acidic (AJJ) and neutral (NIS) soils.
†ST, Tl concentration in the plant shoot; RT, Tl concentration in the plant root; BAF, bioaccumulation factor = TI concentration in shoot / Tl concentration in soil; TLI, translocation index (%) = (TI concentration in shoot / Tl concentration in whole plant) × 100; AJJ, acidic Jeonju soil; NIS, neutral Iksan soil.
The TLI values of metals are obtained from the shoot and root metal concentrations of plants, so that the plant shoot or root metal concentrations are mostly well correlated with the TLI values of the metals. Thus, the Tl concentrations in the shoots and roots of bean plant were significantly correlated (at p < 0.001) with the Tl-TLI values for the plant in both the acidic and neutral soils with the correlation coefficient values ranging from 0.836 to 0.919. Nonetheless, the Tl concentrations in the different parts of cucumber plant tissues were a little or negatively correlated with the Tl-TLI values for the plant. Moderately negative correlations were observed between the shoot and root Tl concentrations and TLI values for the plant with correlation coefficient values ranging from -0.413 (at p < 0.05) to -0.667 (at p < 0.001). According to those results, correlations among the shoot and root Tl concentrations, Tl-BAF values, and Tl-TLI values for bean plant were higher than for cucumber plant.
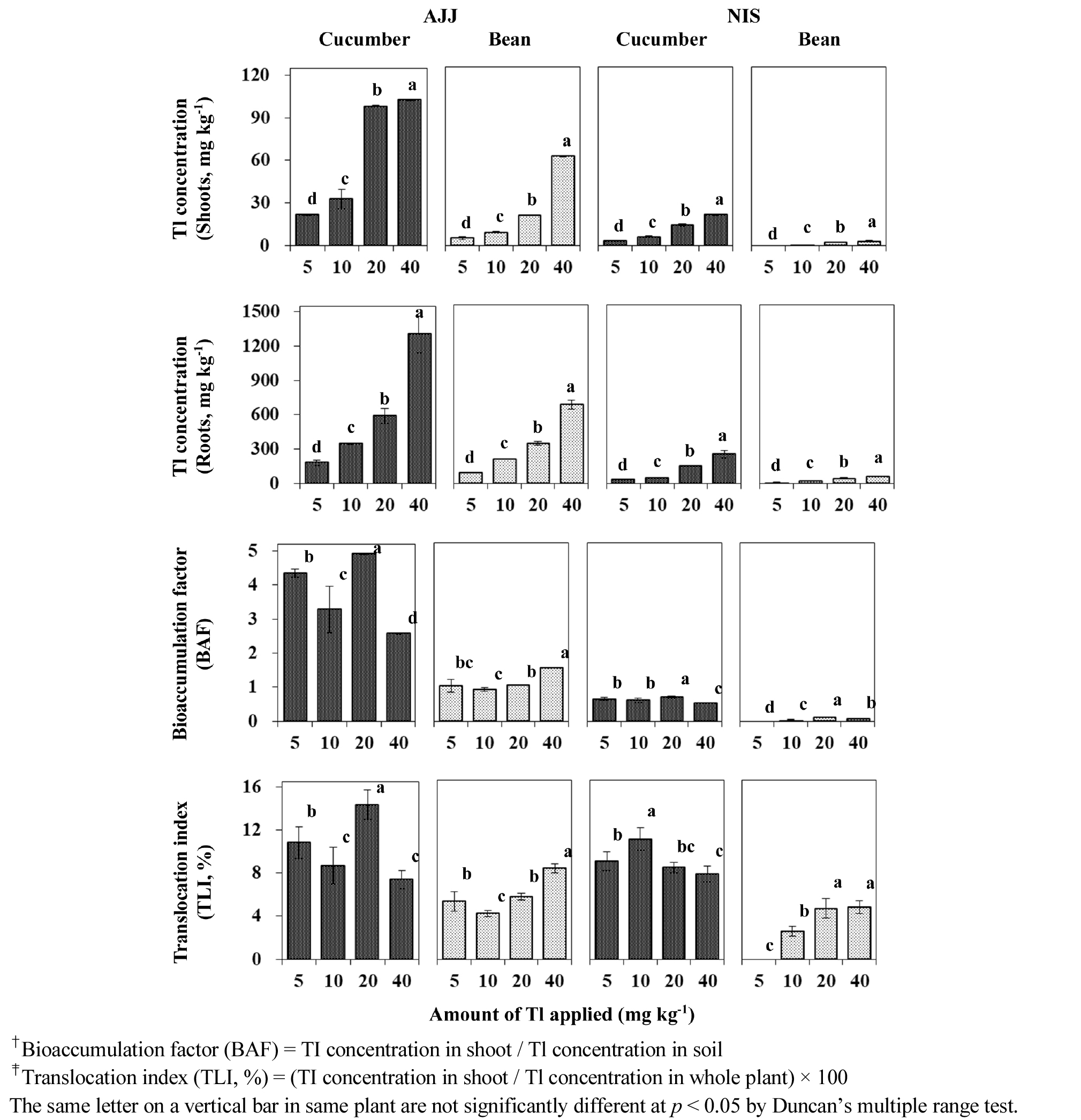
Uptake of Tl by cucumber and bean plants was affected by DTPA treatment in the AJJ and NIS soils (Fig. 2). The concentrations of Tl in the different parts of plants grown in the AJJ soil significantly increased only at the highest DTPA application (30 mg DTPA kg-1 soil), except in cucumber plant roots. In the NIS soil, however, the Tl concentrations in most of the plant tissues increased with increasing the soil DTPA concentration. Significant difference between the shoot and root Tl concentrations of both the plants was observed with the different DTPA concentrations applied in the soil.
In addition, the Tl-TLI values for cucumber plant in the AJJ soil decreased with increase in soil DTPA concentrations. This result might be caused by changes in the shoot and root Tl concentrations. The root Tl concentrations of the plant significantly increased with increasing the DTPA concentrations in the soil, but the plant shoot Tl concentrations were not significantly changed. Besides, the TLI values for bean plant in the AJJ soil increased with increasing the soil DTPA concentrations. In particular, in the NIS soil, the values for both the plants significantly increased because Tl in the aerial part of the plants tended to be more markedly increased with increase in soil DTPA concentration than Tl in the terrestrial part of the plants.
These results showed that DTPA application in NIS soil affected positively the translocation of Tl from the root to the shoot of cucumber and bean plants, whereas DTPA in AJJ soil influenced Tl translocation only in bean plant. Chelating agents including DTPA generally increase the desorption of heavy metals from the soil matrix into the soil water, and also enhance the metal translocation from roots to shoots. The effect, however, may vary from various plants and metals (Yeh and Pan, 2012).
Correlation coefficient (r) relating the uptake and translocation of Tl in cucumber and bean plants influenced by DTPA applications in the different soils is shown in Table 3. When DTPA was applied to the soils, uptake of Tl from the different soils by cucumber plant was statistically different compared with bean plant. The shoot Tl concentration of cucumber plant in the AJJ soil was not correlated (r = 0.181) with the plant root Tl concentration. In the NIS soil, there was relatively low or moderate correlation (r = 0.448 at p < 0.05) between the shoot and root Tl concentrations of the plant. However, the shoot Tl concentrations of bean plant grown in both the acidic and neutral soils were significantly correlated (at p < 0.001) with the root Tl concentrations of the plant, and there were strong positive correlations with correlation coefficient values of 0.938 to 0.911, respectively.
Table 3
Correlation coefficient (r) relating thallium uptake and translocation in cucumber and bean plants grown in the acidic (AJJ) and neutral (NIS) soils as affected by the applications of DTPA.
†ST, Tl concentration in the plant shoot; RT, Tl concentration in the plant root; BAF, bioaccumulation factor = TI concentration in shoot / Tl concentration in soil; TLI, translocation index (%) = (TI concentration in shoot / Tl concentration in whole plant) × 100; AJJ, acidic Jeonju soil; NIS, neutral Iksan soil.
Moreover, the Tl-TLI values for cucumber plant in the AJJ soil were moderately correlated with the plant shoot Tl concentrations (r = 0.499 at p < 0.05), whereas a negative correlation (r = -0.758 at p < 0.001) was observed between the plant root Tl concentrations and Tl-TLI values. In the NIS soil, the shoot Tl concentrations of the plant were significantly correlated (r = 0.949 at p < 0.001) with the Tl-TLI values, but the root Tl concentrations were not correlated with them. However, moderate (r = 0.755 at p < 0.001) or strong correlations (r = 0.916 to 0.997 at p < 0.001) were observed between the shoot or root Tl concentrations and the Tl-TLI values for bean plant in both the soils.
Conclusions
We investigated thallium uptake by cucumber and bean plants that are popular and widely cultivated in Korea. The shoot and root Tl concentrations of the plants increased with increasing Tl concentrations in the acidic (AJJ) and neutral (NIS) soils. The uptake of Tl by cucumber plant was always higher than by bean plant. The root Tl concentrations of both the plants were much higher than the shoot Tl concentrations. The concentrations of Tl in the plant tissues were markedly higher in the AJJ soil than in the NIS soil.
Most of Tl-BAF and Tl-TLI values for the plants did not show a specific trend with different Tl concentrations in the soils. These values only for bean plant grown in the AJJ soil increased with increase in soil Tl concentration. The values were always higher for cucumber plant than for bean plant in both the soils. However, the correlations among the shoot and root Tl concentrations, Tl-BAF values, and Tl-TLI values for bean plant were higher than for cucumber plant.
When DTPA was applied to the soils, the Tl concentrations in most of the plant tissues tended to be increased with increase in the soil DTPA concentration. Significant difference between the shoot and root Tl concentrations of both the plants was observed. In addition, the Tl-TLI values for cucumber plant in the acidic soil decreased with increase in soil DTPA concentrations, whereas the TLI values for bean plant increased. Besides, the values for both the plants in the NIS soil significantly increased with increasing the DTPA concentrations. Thus, the DTPA application in neutral soil affected positively the translocation of Tl from the root to the shoot of both the plants, but the DTPA treatment in AJJ soil influenced the Tl translocation only in bean plant. In addition, further research investigations for Tl uptake by specific hyperaccumulators grown in different soils and comparison between Tl extractability of various chemical extractants from soils and Tl uptake by different plants will be necessary.