Introduction
Materials and Methods
Physico-chemical properties of illite and illite-containing prototype production
Plant materials, treatment and sampling
Analysis of soil and plants
Photosynthetic characteristics
Analysis of total polyphenols and flavonoids
Determination of antioxidant activities
Experimental design and statistical analysis
Results and Discussion
Effects of illite-containing prototype (A) on soil chemical properties
Effects of illite-containing prototype (B) on plant growth
Introduction
Clay minerals are well known to improve physical (i.e., water retention) and chemical (i.e., cation exchangeable capacity) properties of soils. Therefore, an agricultural use of clay minerals including illite have gradually been enlarged to take advantages like soil amendment and crop growth promotion (Zhang and Park, 2007). Illite is a non-expanding clay mineral which is structured by 2:1 of silica-alumina-silica layer, and contains high potassium (K) (Barré et al., 2006). Illite is generally considered to have a relatively low capacity to hold and release exchangeable ions such as Ca, Mg and Na, but a high potential to accumulate K into the soils (Barré et al., 2006). A majority of clay minerals including illite have been extensively examined to elucidate their effects on soil amendments, especially heavy metal-derived soil toxicity (Yin et al., 2015). Potassium (K), which is the most abundant cation accounting up to 10% on dry weight basis, plays important roles in plant growth and development (Leigh and Jones, 1984). Supplemental K application enhances the enzymatic and non-enzymatic antioxidative activities and plant growth. Moreover, K-fed plants significantly increase the synthesis of osmolytes such as free proline, amino acids and sugars (Ahanger and Agarwal, 2017). Besides, a huge number of enzymes is either absolutely dependent on or stimulated by K, and contributes to cell elongation and osmotic adjustment (Bhandal and Malik, 1988). The adequate K application could enhance primary and secondary metabolites including antioxidative compounds (Nava et al., 2007; Liaqat et al., 2012; Zareei et al., 2018), and increased total phenols, flavonoids and antioxidant activity (DPPH, FRAP) in tomato leaves (Chrysargyris et al., 2017) and basil (Salas-Pérez et al., 2018). Therefore, it is carefully considered that an illite including high K might also enhance antioxidative activities as applied into the soil. All things taken together, it is expected that high K-containing illite has direct and/or indirect effects on soil amendment and plant growth promotion. The aim of this study is to investigate the effects of illite-containing fertilizer prototypes for soil amendment and crop growth on the basis of physico-chemical properties of natural illite. To do these goals, we investigated the effect of the application of different types of illite-containing fertilizer prototype, soil ameliorant and plant growth promotion.
Materials and Methods
Physico-chemical properties of illite and illite-containing prototype production
An illite was mined at Youngdong-gun, Chungbuk province, and physico-chemical properties were analyzed (Table 1). The silicon dioxide (SiO2) and aluminium oxide (Al2O3) were major chemical components, and, as physical properties, the thickness, diameter, base space and particle density were 30 Å, 10 Å and 2.6 g cm-3. Suitable microbe and a bonding agent were selected based on prior to developing a prototype for soil amendment and crop growth promotion, and some properties such as plasticity, collapse and hardness were measured. The materials and combination to develop the illite-containing fertilizer prototypes were shown in Table 2, and the prototypes produced were represented in Fig. 1.
Table 1
Physico-chemical properties of illite used in this study.
| Properties | Physical | Chemical (%) | ||||||||
|
Thickness (Å) |
Diameter (nm) |
Base space (Å) |
Particle density (g cm-3) | SiO2 | Al2O3 | Fe2O3 | K2O | CaO | TiO2 | |
| 30 | 0.1 - 0.3 | 10 | 2.6 | 57.5 | 23.3 | 6.76 | 6.43 | 0.1 | 0.38 | |
Table 2
Materials and combination for illite-containing fertilizer prototypes.
Plant materials, treatment and sampling
Seeds of tomato (cv. Superdotaerang) were sown in a plug pot (128-holes), and uniformly growing seedlings were selected and transplanted into plug pots (2 × 36-holes) at 2 weeks after sowing and grew further 2 weeks for an experimental use. Four-weeks-old tomato plants were transplanted into plastic containers (20 cm width × 30 cm height) including soils of 15 kg. Two types of treatment were subjected to this study; 1) control, dolomite limestone, illite and prototype-A for soil amendment and 2) control, illite, NPK and prototype-B for plant growth promotion (Table 2). Firstly, an experimental soil (see the Table 3 for chemical property) was mixed with dolomite limestone, illite, prototype-A or prototype-B prior to 5 days of transplanting. The application rate of dolomite limestone, illite, prototype-A and -B was adjusted to 2,000 kg ha-1. A treatment of chemical fertilizer (NPK) was estimated on the basis of soil testing (N, 204 kg ha-1; P, 103; K, 122). N (urea) and K (potassium chloride) were split as top (60%) and side (40%, with 3 splits) dressing, and P (monopotassium phosphate) was applied as a basal dressing. Soils from each treatment were carefully taken for the analysis of soil chemical properties (n = 3). Tomato plants displaying the similar growth (n = 5) within each treatment (n = 8) were taken between 10:00 - 12:00 to avoid diurnal effects on the physiological changes at 60 days after the initiation of treatment. After harvest, leaves, stems and fruits were briefly rinsed with deionized water and stored at -80°C prior to the measurement of antioxidative activities after freezing in liquid nitrogen or oven-dried at 80°C for 48 h for mineral nutrient analysis.
Table 3
Effect of illite-containing soil ameliorant (prototype-A) application on soil chemical properties.
Analysis of soil and plants
The chemical analysis of soil and plant was followed by the recommended methods (Lee et al., 2017a, 2017b). In order to measure soil chemical properties (n = 3), the fresh soil samples (5 g) were extracted with 25 mL of ddH2O for pH and electric conductivity (EC) and 25 mL of 2 M KCl for 30 min for inorganic N (nitrate- and ammonium-N), filtered, and measured with a pH/EC meter and nitrogen analyzer (440 nm, Bran+Luebbe, Germany), respectively. For the measurement of available P2O5, the dried soil samples (5 g, <0.053 mm) were extracted with a lancaster buffer (pH 4.25) and measured with a UV-spectrophotometer (720 nm, UV-2450, Shimadzu, Japan) after the filtration (Whatman No. 2). The exchangeable cations of dried soil samples were measured with an ICP (INTEGRA XMP, GBC, Australia) after the extraction (1 N CH3COONH4, pH 7.0) and filtration (Whatman No. 2), and a cation exchange capacity (CEC) was calculated with the method (NIAST, 2010). In order to analyze mineral nutrients of plants (n = 5), the powdered samples (100 mg, DW), leaves, stems and fruits, were mixed with 5 mL of 368 mmol-1 L salicylic acid in 84.7% sulfuric acid (H2SO4, v/v) for 24 h and wet-digested at 300°C for 6 h, followed by the addition of several drops of hydrogen peroxide (H2O2). The extract was transferred to a 100 mL volumetric flask and diluted to 100 mL with deionized water for the mineral assays. Nitrogen concentration was determined using an automatic flow injection analyzer (Bran+Luebbe, Germany). Phosphorus concentration was measured using the molybdate-blue colorimetry method using a UV spectrophotometer (UV-2450, Shimadzu, Japan). The cations including K, Ca, Mg, Fe, Cu, Mn and Zn, were measured using an ICP-OES equipment (INTEGRA XMP, GBC, Australia) according to the manual.
Photosynthetic characteristics
A portable photosynthesis device (ADC LCpro+, ADC BioScientific Ltd, UK), equipped with a square chamber (6.25 cm2), was used for measurements of CO2 fixation (A), transpiration rate (E) and stomatal conductance (gs). The quantum flux density from solar radiation at the time of assay was 800 ± 50 µmol m-2 s-1 and the CO2 level was 400 ± 10 µmol. The measurement was performed from 10:00 am to 14:00 pm to avoid a marked variation in photosynthesis occurring after midday.
Analysis of total polyphenols and flavonoids
The freeze-dried powdered tomato leaves (0.2 g) were extracted with 15 mL of 70% EtOH for 0.5 h with 200 rpm of shaking. The extracts were centrifuged, filtered through 0.45-µm filters, and measured with slight modification of Folin-Ciocalteu method (Folin and Denis, 1912). Briefly, 250 µL of the aliquot was mixed with 5.0 mL distilled water including 2% of Na2CO3, reacted for 30 min at darkness after an addition of 250 µL of Folin-Ciocalteu reagent. The absorbance of the extracts was measured at 750 nm. The different concentrations (0 - 500 µg mL-1) of gallic acid were used as the standard and the results were expressed as µg gallic acid equivalents (µg GAE g-1) of fresh weight. For measurement of total flavonoids (Moreno et al., 2000), after same extraction, 500 µL of the extracts was mixed with 2.0 mL distilled water and 150 µL of NaNO2, 300 µL of 10% AlCl3·6H2O was added into the mixture, and, finally, 1,000 µL of 1 M NaOH was added. The absorbance of the extracts was measured at 510 nm. The different concentrations (0 - 500 µg mL-1) of catechin were used as the standard and the results were expressed as µg catechin equivalents (µg g-1) of fresh weight.
Determination of antioxidant activities
The freeze-dried powdered tomato leaves (0.2 g) were extracted with 15 mL of 70% EtOH for 0.5 h with 200 rpm of shaking. The extracts were centrifuged, filtered through 0.45-µm filters, and used to determine antioxidant activities with two assays; 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH). The ABT assay was measured with slight modification of the method (Re et al., 1999). Firstly, the ABTS radical cation (ABTS+) was produced by reacting 7.4 mM ABTS solution with 2.6 mM potassium persulfate, stood in darkness for 24 h, and then diluted with distilled water to an absorbance of 1.0 ± 0.02 at 735 nm before use. The extract (200 µL) was mixed with 4 mL of ABTS+ solution, and the absorbance was measured at 735 nm after 0.5 h of dark incubation. Free radical scavenging activity was measured using the DPPH assay using the method (Blois, 1958). The aliquot (750 µL) was mixed with 3 mL of 0.2 mM DPPH solution. After darkness for 0.5 h, the absorbance was measured at 525 nm. The result was expressed as a percentage (%) of scavenging activity.
Experimental design and statistical analysis
The experimental design was followed by the completely randomized design (n = 8), and, of those, soils (n = 3) and plant (n = 5) were taken for the analysis. Statistical analysis for all measurements was performed using SAS software (version 9.2). Data were subjected to one-way ANOVA. If the ANOVA yielded a significant F value (p < 0.05), the differences between treatments were compared using Tukey’s range test.
Results and Discussion
Effects of illite-containing prototype (A) on soil chemical properties
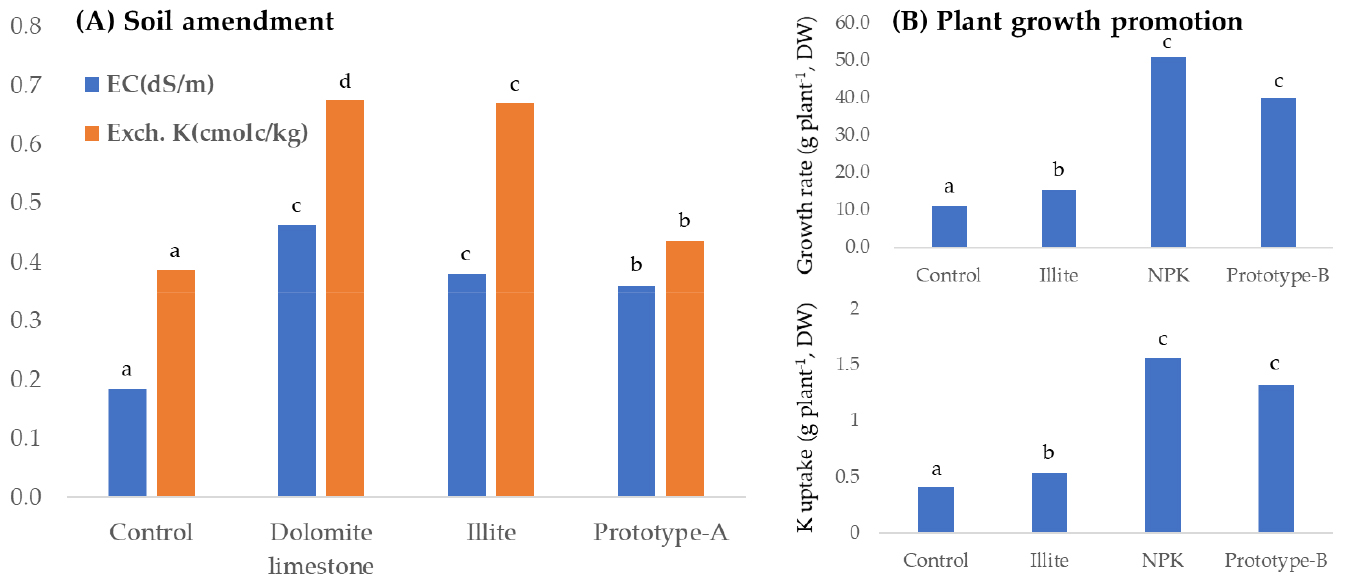
After 60 days of treatment, the rhizosphere soils were carefully obtained from each treatment (n = 3); control, dolomite limestone, illite and prototype-A. As shown in Table 3, an application of illite resulted in an increase in EC (p < 0.05), however, decreased soil pH. In contrast, prototype-A showed significant decrease in soil pH (from 6.3 to 5.5) and increase in EC (from 0.2 to 0.4) (p < 0.05). These findings are consistent with the previous studies examining different types of illite on soil chemical properties (Kim et al., 2010; Lee et al., 2010). Additionally, the illite treatment showed a dramatic increase in CEC (p < 0.05), whereas a prototype-A containing illite was not significantly differed from the control. It is presumably considered that sugar and microbes added as a binding agent and enhancer led to the decrease in soil pH, and thus lower pH limited an increase in CEC. An application of organic and inorganic fertilizers resulted in an obvious decrease in soil pH due to chemical and biological reactions (Kang et al., 2004; Pyakurel et al., 2019; Yousfi et al., 2021). In this context, the substrates such as sugar and microbes are responsible for decreasing soil pH, thereby releasing organic acids which are the decomposed products by microbes. According to our results, a prototype-A is believed to contribute to lowering soil pH and increasing EC. Therefore, we concluded that a prototype-A could be used as a possible soil ameliorant to adjust an alkaline soil with the supply of K into soil.
Effects of illite-containing prototype (B) on plant growth
A prototype-B (illite-containing fertilizer) enhanced growth rate (dry weight-based per plant) and the uptake of some of macronutrients in tomato plants (leaves + stems + fruits) obtained at 60 days after treatment (p < 0.05) (Table 4). The growth rate of tomato plants was about 4 times greater than the control, and the uptake of N, P, K, Ca and Mg was 3.2 to 4.3-fold increase (4.3, 3.2, 3.2, 4.1 and 3.7, respectively) compared to the control. Given that the results from a NPK treatment, a prototype-B showed obvious effects on the uptake of N, P, K and Ca, which was not significantly differed from a NPK treatment, although the growth rate was lower in a prototype-B. Our results were in line with previous observations from pepper (Lee et al., 2010, 2012a, 2012b, 2014) and cherry tomato (Kim et al., 2010). In a view of a practical use, it is suggested that a prototype-B could certainly improve plant growth and mineral uptake.
Table 4
Application effect of illite-containing fertilizer (prototype-B) on the growth and nutrient uptake† of tomato plants.
The photosynthetic characteristics measured at 60 DAT was positively related to an application of a prototype-B (Table 5). An intercellular CO2 concentration, an indicator of CO2 consumption by CO2 fixation reaction in the mesophyll, showed a significant reduction, which was about 27% lower compared to the control. Additionally, CO2 fixation rate in a prototype-B-applied tomato plants was 8.7 µmol m-2 s-1, which indicated about 3.3 times greater (p < 0.05) than the control and similar level to NPK treatment, whereas transpiration and stomatal conductivity were not differed between treatments. Many evidences have been reported that increasing or optimized N supply affects the N content in leaves, which is mainly located in the photosynthetic protein complex, thereby enhancing photosynthesis (Vos et al., 2005; Liu et al., 2019; Yue et al., 2021). Given that previous observations, a prototype-B is considered to influence positively the photosynthesis, and is followed by enhancing dry weight. We also examined some of antioxidant activities such as polyphenols, flavonoids, ABTS+ and DPPH, which were known to reduce an oxidative damage under biotic and abiotic stresses, from leaves and fruits tomato plants (Table 6). A prototype-B affected positively total polyphenols and flavonoids, which showed about 26 and 35% increase (p < 0.05) compared to the control, in the leaves, whereas the ABTS+ and DPPH were not differed from the control. In the fruits, total polyphenols and ABTS+ were significantly increased by a prototype-B treatment, whereas total flavonoids were lower compared to the control (p < 0.05). A previous study (Lee et al., 2014) also observed that an application of illite resulted in the significant increase in some of antioxidants from Saururus chinensis leaves, and this was in line with our finding. Taken together, it is expected an obvious enhancement of some of antioxidants in a prototype-B application is a synergistic effect of illite and chemical fertilizer. Therefore, one possible assumption could be carefully suggested that higher growth (Table 4) in a prototype-B application led to more soluble carbohydrate synthesis, expressed as a photosynthesis, (Table 5), and an activated translocation of photoassimilates from leaves to fruits resulted in secondary metabolites (antioxidants) (Table 6).
In conclusion, the application of a prototype-A, illite- and microbes-containing soil ameliorant, showed positive effects on an abundance in soil chemical properties such as EC and exchangeable K, and is also observed to be lowered in soil pH although further survey is strongly required. In addition, a prototype-B, illite- and NPK-containing fertilizer for plant growth promotion, also showed to have positive effects on tomato growth and antioxidant activities. Therefore, illite-containing prototypes for agricultural use could be helpful to enhance soil chemical quality and plant growth.