Introduction
Materials and Methods
Experimental setup and treatment
Sampling, growth parameters and C/N analysis
Extraction and analysis of polar compounds
Statistical analysis
Results and Discussion
Rice growth and nutrients (C and N) uptake
Metabolites in the shoot and grain
Conclusions
Introduction
Food waste of 5.8 MT per year is produced nationwide, indicating an occupancy of 30% of total wastes (ME, 2019), and this is of great concern in this era of environmental degradation to handle in an agricultural sector. Due to food waste-derived environmental issues, several developed countries recognized food waste as one of alternative resources, and, therefore, have tried to recycle and/or reuse through an improvement of composting technologies (Glenn and Goldstein, 1999; Touart, 1999; Sikora and Sullivan, 2000).
Despite an abundance in essential nutrients including nitrogen (N) for crop growth, the relatively higher water content (>80%) is a difficulty to directly incorporate into crop lands due to the generation of hazardous gases such as methane and hydrogen sulfide during the decomposition (So et al., 2007). Nevertheless, the demand of an agricultural application of food waste is constantly increasing, and thus research projects and composting facilities by central/local governments are being enlarged to find the possibility of food waste-derived compost as an alternative. Food waste-derived composts after the composting process include 4, 2 and 1% of nitrogen (total N), phosphorus (P2O5) and potassium (K2O), respectively, and contribute favorable crop growth and yield enhancement (Lee et al., 2016). However, higher NaCl in food wastes was proposed as a problem to lead to salt stress of crops and the contamination of soil and ground water (Gerber et al., 2005). Therefore, many studies have tried to identify the customized application rates as well as the effect of crop production from economic crops including rice (Lee et al., 2016; Cho et al., 2021), pepper (Lee et al., 2016), cucumber, tomato (Yoo et al., 2018), Chinese cabbage (Yoo et al., 2018; Kim et al., 2021) and orchardgrass (Cho et al., 2000).
The N is the most important element requiring large quantity for crop plant including rice, and plays a pivotal role in numerous cellular metabolisms. Despite of its importance ensuring crop yield, excessive N use frequently acts a driving force to cause environmental problems including ground water contamination. The soluble carbohydrates (sucrose) and N compounds (mainly asparagine) are translocated/redistributed form leaves to grains during the grain filling stage (Osaki et al., 1991, 1992). The N dressing within the optimal range has a positive effect on grain protein accumulation due to producing amino acids at the panicle development stage (Perez et al., 1996; Borrell et al., 1999), and free amino acids in rice grain tends to a tendency of increase (Ning et al., 2010). On the other hand, some studies with high N input observed the reduction in non-structural carbohydrates and amino acids during rice grain filling, which means relatively greater vegetative growth (Yang et al., 2001; Hirano et al., 2005; Jiang et al., 2016). The comparative metabolite profiling was proven to provide valuable information in various fields of plant science including crop quality assessment (Hall et al., 2005) and environmental impacts (Röhlig and Engel, 2010). However, little information is available concerning the metabolite composition between organs at relative variable time like heading stage due to mainly based on analysis of mature products, and, moreover, the dynamic changes in metabolomic processes of the leaf and grain at the heading stage have not well been investigated in rice plants fed with excessive rates of fertilization.
Therefore, we hypothesized that greater N from an additional and increasing input of FW-LMC could result in a perturbation of organ-specific primary metabolism. In this study, we aimed to gain a better understanding how an additional FW-LMC affected primary metabolism in the leaf blade and grain of rice plant at the heading stage. To achieve our goal, we examined metabolite profiling using GC-TOF/MS.
Materials and Methods
Experimental setup and treatment
This study was carried out at the experimental paddy field located in Chungbuk national university, and each plot was designed with 16.0 m (width) × 3.6 m (length). The physico-chemical properties used for this study were described at Table 1. The experimental field was flattened prior to the application of fertilizer sources. The study consisted of six treatments as follows: Non fertilizer; 100% Chemical fertilizer of standard recommendation (SF); 100% CF of soil diagnosis recommendation (SDF); 100, 150 and 300% compost made of food waste and livestock manure (FW-LMC) as an alternative N by soil-diagnosis.
The seeds of rice cultivar ‘Saechucheongbyeo’ after an imbibition for 48 hr were germinated in a plastic container, and the uniformly growing 20-days-old seedlings were transplanted with a planting density of 12 cm (between hills, 4 plant hill-1) × 20 cm (row). The application rates from standard and soil diagnosis of NPK (urea for N; superphosphate for P; potassium chloride for K) was 90:45:57 and 87:78:28 kg ha-1, respectively, and fertilized at 3 days before transplanting. The FW-LMC was supplied with the rates of 10 (100%), 15 (150%) and 30 (300%) Mg ha-1 as a basal dose at 15 days before transplanting, and an additional dose of N and K was equivalent to soil diagnosis recommendation. N was applied with three splits (58% as basal, 22% at tillering, and 22% at panicle initiation), potassium with two splits (70% as basal and 30% at panicle initiation). The practices for rice cultivation such as irrigation, insecticide and herbicide followed the rural development administration (RDA, Korea) guide.
Table 1.
Physicochemical properties of soil used in this study.
Sampling, growth parameters and C/N analysis
The uniformly growing rice plants (n = 6) were harvested at heading stage, some agronomic parameters were measured, and they were carefully divided into shoots and grains for further analysis. The dried samples (0.1 g) were put in the quartz crucibles, which was provided by manufacturer, and were combusted at high temperature (1,000°C) (CN auto-analyzer, Primacs SNC-100, SKALAR, Netherland). Total C and N were measured by high temperature combustion method with non dispersive infrared detection (NDIR) and by Dumas method with thermal conductivity detection (TCD), respectively.
Extraction and analysis of polar compounds
Polar compounds were identified with Kim’s protocol (Kim et al., 2016). One hundred milligrams of powdered samples were extracted with 1 mL of methanol : water : chloroform (2.5:1:1, v/v/v) solution, and ribitol (60 µL, 0.2 mg mL-1) solution was used as an internal standard (IS). Extraction was performed at 37°C at a mixing frequency of 1,200 rpm for 30 min using a Thermomixer Compact (Eppendorf AG, Germany). The extracts were centrifuged at 16,000 × g for 3 min. The polar phase (0.8 mL) mixed with 0.4 mL water was centrifuged at 16,000 × g for 3 min. The methanol/water phase was dried in a centrifugal concentrator (CC-105, TOMY, Tokyo, Japan) for 2 h, followed by a freeze dryer for 16 h. MO-derivatization was performed by adding 80 µL of methoxyamine hydrochloride (20 mg mL-1) in pyridine and shaking at 30°C for 90 min. TMS-esterification was performed by adding 80 µL of MSTFA, followed by incubation at 37°C for 30 min. GC-TOFMS was performed using an Agilent 7890A gas chromatograph (Agilent, Atlanta, GA, USA) coupled to a Pegasus HT-TOF mass spectrometer (LECO, St. Joseph, MI). Each derivatized sample (1 µL) was separated on a 30-cm × 0.25-mm I.D. fused-silica capillary column coated with 0.25-µm CP-SIL 8 CB low bleed (Varian Inc., Palo Alto, CA, USA). The split ratio was set to 1:25. The injector temperature was 230°C, and a flow rate of helium gas through the column was fixed with 1.0 mL min-1. The temperature was set up as follows: initial of 80°C for 2 min, followed by an increase to 320°C at 15°C min-1 and a 10 min hold at 320°C. The transfer line temperature and ion-source temperature were 250 and 200°C, respectively. The scanned mass range was 85 - 600 m/z, and the detector voltage was set to 1,700 V. ChromaTOF software was used to support peak findings prior to quantitative analysis and for automated deconvolution of the reference mass spectra. NIST and in-house libraries for standard chemicals were utilized for compound identification. The calculations used to quantify the concentrations of all analytes were based on the peak area ratios for each compound relative to the peak area of the IS.
Statistical analysis
An analysis of variance was performed to compare the differences between treatments using a statistical program (SAS ver. 9.4). Data were analyzed in a completely planned plot design using one-way ANOVA, and, if p < 0.05, were subjected with Duncan’s multiple range test (DMRT) to detect significant differences among the means.
Results and Discussion
Rice growth and nutrients (C and N) uptake
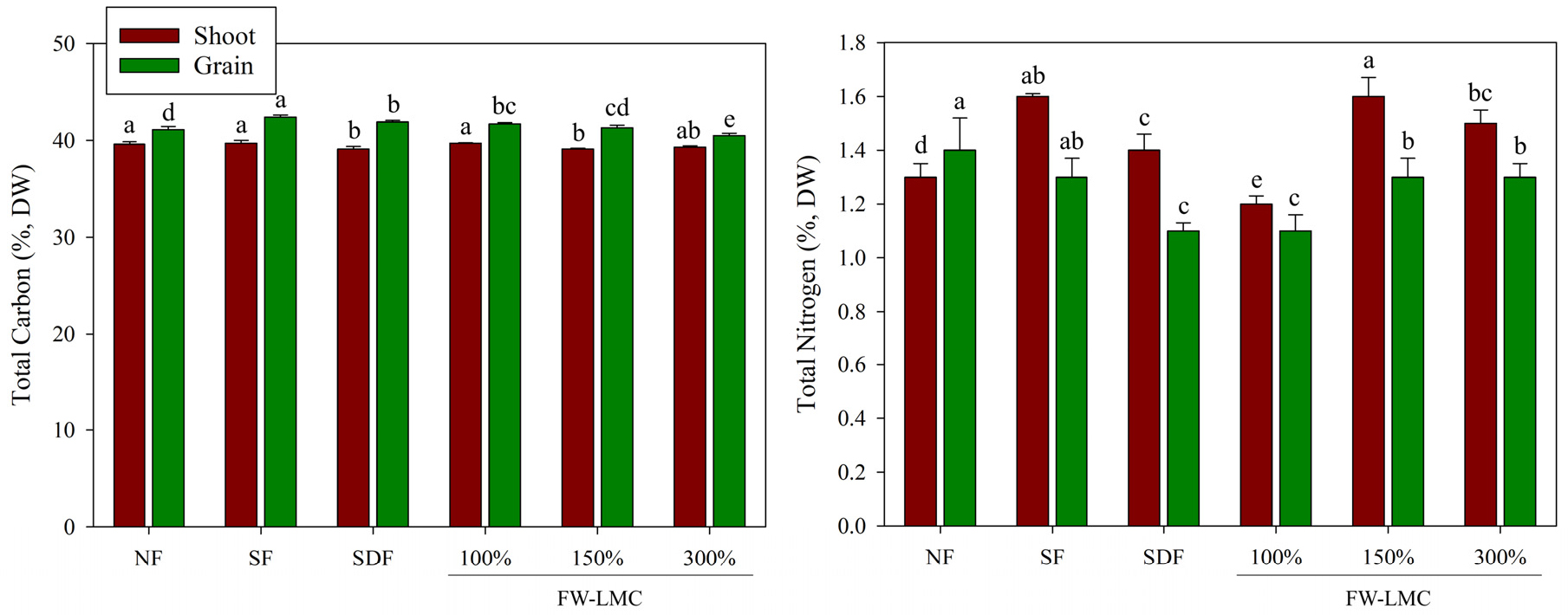
The greater application rates of nitrogen source resulted in the significant increase in some agronomic parameters such as plant height, dry weight and tillers (Table 2). The highest enhancement of plant height (120 ± 2.0 cm) and shoot dry weight (61.0 ± 4.3 g hill-1) of rice plants was observed in the SDF + 150% of FW-LMC treatment, which is indicating 1.2 and 1.8-folder greater compared to NF treatment. In contrast, higher application rates of nitrogen source didn’t show any effect on grain weight. The remarkable increase in tiller by higher nitrogen input significantly produced the reproductive tillers, which affect the number of panicles. The greater input, a critical point to avoid crop damage, of FW-LMC-based fertilization significantly promoted biomass production in rice (Cho et al., 2021), red pepper (Kim et al., 2020) and Chinese cabbage (Kim et al., 2018, 2021) although greater and longer input could lead to the enhancement of soil EC to cause unfavorable crop production (Yoo et al., 2018; Kim et al., 2019, 2021; Lee et al., 2019). Therefore, the current result provides a possibility that higher input of nitrogen source, especially an organic resource such as FW-LMC, in rice cultivation system could markedly enhance the vegetative growth as well as the reproductive production. Total carbon (C) and N in the shoots and grains of rice plants taken at heading stage represented significant differences between treatments (Fig. 1). The SDF and FW-LMC 150% showed the lowest total C in shoots compared to other groups, whereas the lowest total N was observed at FW-LMC 300%. In contrast, the highest total N in shoots was in the SF and FW-LMC 150%, and, for grains, was in the NF and SF. The higher N content in leaves might result from concentrated nitrate content (Geiger et al., 1998). Subsequently, the increase in N content led to reduced plant C/N ratio under high N fertilization (Ibrahim et al., 2011). N remobilization was quickly initiated after anthesis, as indicated by decreases in the nitrogen and/or protein contents of the leaves as a result of leaf senescence (Kong et al., 2016). Thus, the highest N in grains in the NF was considered as a result of accelerated N remobilization from the source to the sink organ.
Table 2.
Effect of additional application of food waste-livestock manure compost on rice growth (n = 6).
Metabolites in the shoot and grain
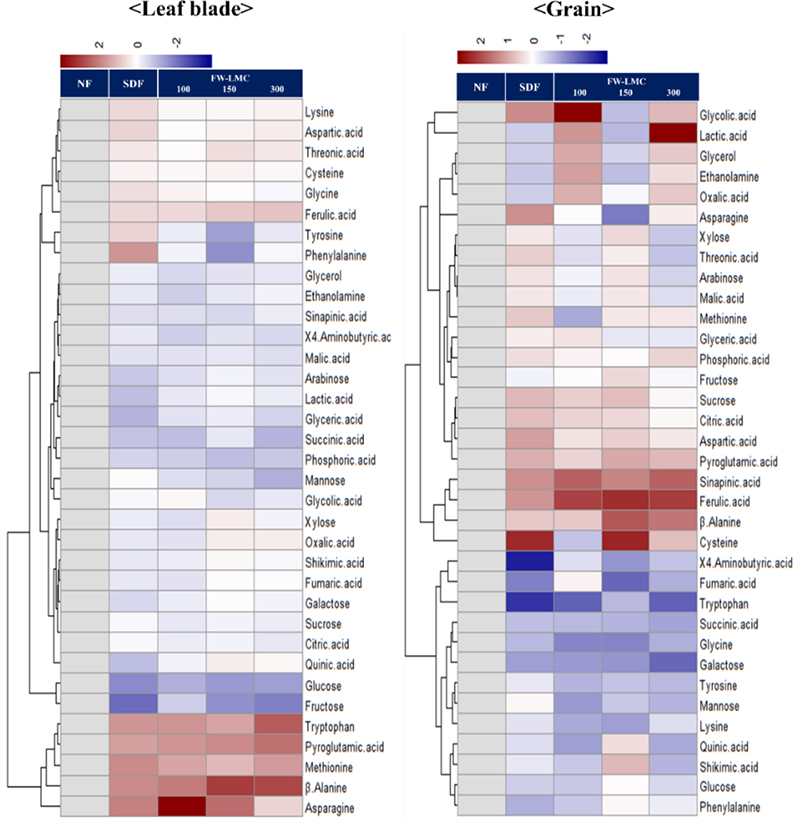
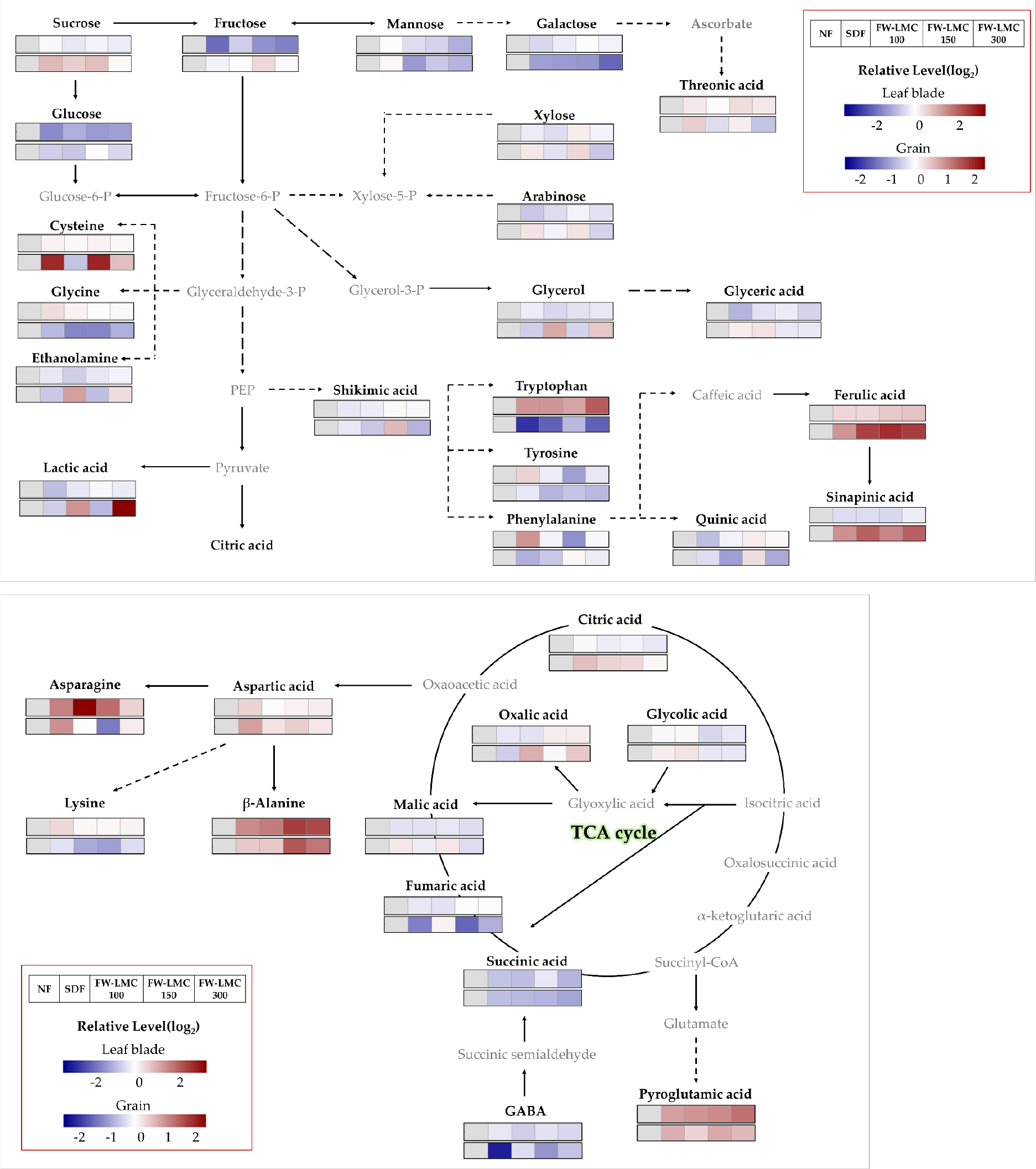
The targeted primary metabolites and intermediates for secondary metabolism were profiled by GC-TOFMS. The relative level (log2 scale) of each treatment compared to the NF was depicted as a heatmap (Fig. 2), and the statistical difference between treatments was presented in Table 3. An abundance in most of metabolites was significantly affected by the SDF and FW-LMC application. Nearly all of soluble carbohydrates significantly declined in the leaf blade, and the tendency of a decrease was remarkable in glucose and fructose. In the grain, an abundance was greatly differed from the type of soluble carbohydrates, which is indicating that glucose, mannose and galactose tended to decrease with a fertilization, whereas sucrose showed significantly greater. The SDF and FW-LMC treatments significantly enhanced the relative level of some amino acids, pyroglutamic acid, asparagine, β-alanine and tryptophan, in the leaf blade. Pyroglutamic acid, aspartic acid, β-alanine and cysteine in the grain were markedly accumulated by the fertilization treatments, whereas the relatively level of glycine, lysine, tryptophan, tyrosine and GABA were greatly decreased. Ferulic acid and sinapinic acid, an intermediate of secondary metabolism, in the grain showed the significantly greater level compared to the NF. Considering the increase in soluble carbohydrates with decreasing fertilization (Amin et al., 2007), the strength of fertilization was oppositely related with the level of soluble carbohydrates, and similar result was demonstrated by other researchers (Den-Hertog et al., 1996; Poorter et al., 1997; Tissue et al., 1997; Ibrahim et al., 2011). Moreover, it was observed that a substantial sucrose translocation from the leaves to grains took place in during the heading stage. The composition of free amino acids greatly depends on sources, rates and types of nitrogen fertilization (Alehina, 1992), and asparagine and glutamine were recognized as an efficient molecule for the storage and transport of nitrogen (Lea et al., 2007). Accordingly, with the previous observations (Weber et al., 2008; Neuberg et al., 2010), our result demonstrated that the relatively higher level of asparagine and pyroglutamic acid in the leaf blades was a storage of nitrogen by greater input of fertilization. Interestingly, our findings in this study revealed that the fertilization resulted in huge accumulation of amino acids like pyroglutamic acid and β-alanine in both organs, and tryptophan accumulation showed the opposite tendency between shoots and grains.
Table 3.
Statistical difference between treatments for the metabolites measured from the leaf blades and grains of rice plant.
Conclusions
In current study, we have investigated some agronomic parameters and a variation of primary metabolites from the leaf blade and grain of rice plants harvested at heading stage. In conclusion, an additional application of the FW-LMC resulted in the remarkable vegetative growth of rice plants. Total N in grain by increasing FW-LMC rates could result in lower grain quality at harvest due to greater accumulation of protein. In terms of primary metabolism, most of soluble carbohydrates showed to be less accumulated due to higher N input from the FW-LMC, an abundance of amino acids greatly depended on the organ, leaf blade and grain. Accordingly, the addition of FW-LMC in chemical fertilizations (SF and SDF) could lead to marked perturbations in primary metabolism, and these variations contributed greater vegetative growth of rice plants, whereas could be a factor to reduce grain quality. Finally, we carefully suggest a necessity of further study on how the greater input of fertilization like the FW-LMC contributes grain qualities such as protein and amylose contents at the harvest stage.