Introduction
Red pepper (Capsicum annuum L.) is a crop belonging to Solaceae family and is one of the most consumed vegetable crops in Korea. Most red peppers are consumed throughout the year as dried pepper or red pepper powder. Pepper is a condiment used mostly for kimchi and red pepper paste, and has been widely used, making it an important place in Koreans' diet (Guang-Cheng et al., 2010; Hwang et al., 2011). The cultivation of peppers takes place mainly in the bare ground, and the area was surveyed at 28,824 ha in 2018 (MAFRA, 2018). Recently, pepper cultivation in the bare ground has been gradually decreasing, and facility cultivation with high production per unit area has been increase (Lee et al., 2004; Yang et al., 2011). While intensively growing peppers, excessive use of the same fertilizers, compost and organic materials in the same place results in a nutrient imbalance in the soil (Park et al., 2009).
When growing crops in a facility to increase income of a farming household, a large amount of fertilizer is applied into the soil to increase crop yield. Moreover, due to the low price, the convenience of handling, and the high efficiency of the use of nutrients of chemical fertilizers (Hazra, 2016), farmers rarely use other applying methods, such as green manures. However, long-term use of chemical fertilizers will impair the physical and biological properties of the soil and worsen the overall soil environment (Horrigan, 2002; Sun et al., 2015).
The effective way to reduce the use of chemical fertilizers and improve soil properties is known to utilize green manure crops (Reganold et al., 1990; Yadav et al., 2000). The legume family crops as green manure have the advantage of conserving large amounts of nitrogen through biological processes, making it available to the next crop grown (Shipley et al., 1992; Allen et al., 2011). Seasonally advantageous green manure crops include legume crops with high nitrogen fixation in summer, and especially hairy vetch (Vicia villosa Roth) in winter (Clark et al., 1997; Lee et al., 2006; Alam et al., 2015). In order to grow crops efficiently by combining crops and green manure crops, the nutrient balance and inorganic matter circulation is important. In addition, it is necessary to quantify the input of the nutrients supplied for crop cultivation and the absorption of the nutrients removed by the harvested crop products. If the correct nutrient balance can be calculated, it can efficiently manage soil nutrients and realize sustainable farming methods (Bindraban et al., 2000; Naresha et al., 2017).
When using green manure crops, to promote crop production, soil microbial activity needs to consider together. Microorganisms' activity affects soil properties such as carbon or nutrient net fluxes in the long term (Franzluebbers et al., 1994; Wardle et al., 1999). Past studies have confirmed that soil microbial activity was facilitated by soil management activities such as the use of green manure crops or crop rotation (Böhme et al., 2005; Yusuf et al., 2009). Enzyme activity in the soil environment is considered a major cause of the overall soil microbial activity and soil quality (Roldán et al., 2005; Liu et al., 2010; Selvakumar et al., 2018a). For this reason, green manure crop incorporation methods should be assessed to reduce the use of chemical fertilizers, increase soil nutrients and microbial activity, and increase crop production.
The objectives of this study were to investigate the growth characteristics and yields, to analyze the nutrient utilization efficiency, and to measure the effect of soil microbial activity through enzyme activity when incorporating green manure crops with facility-grown peppers.
Materials and Methods
Facility Specification for Plant Cultivation and Treatment Details The experimental facility consisted of 12 blocks was constructed in 2015 in the research fields of the National Institute of Horticultural and Herbal Science, Wanju, Korea (35.8357°N, 127.0350°E). The size of each block was 4 × 3 m. The blocks were separated by 1 m concrete wall to prevent nutrient mobilization to adjacent blocks or leaching out. Subsoil from the non-cultivated demolished hummock was used to fill up the experimental blocks. The chemical properties of the soils in each treatment were measured in April 10, 2018 and the data given in Table 1.
Table 1. Soil chemical properties of experimental blocks before and after red pepper cultivation.
†Each value represents the mean of 3 replications ± standard error.
§Each value in a column followed by the same letter are not significantly different by Duncan's multiple range test at p ≤ 0.05.
Four different fertilizer treatments were applied in the experimental blocks. Each treatment contained three replications in a randomized complete block design. The treatments were as follows: no fertilizer (CON), chemical fertilizer (CF), hairy vetch and 0.5N additional fertilizer (HV0.7N), and hairy vetch and N additional fertilizer (HVN). For chemical fertilizers, conventional synthetic nitrogen (N), phosphorous (P), and potassium (K) fertilizers were applied based on the nutrient requirements recommended by the Rural Development Administration of Korea for red pepper plants. The amount of N, P2O5, and K2O applied for CF treatment were 280.0, 90.0, and 130.0 kg ha-1, respectively (Table 2). For HV0.7N and HVN treatment, hairy vetch seeds were sown in their respective blocks from November to early April of the following year. Hairy vetch was sampled and analyzed for nutrient contents. The amount of N, P, and K in hairy vetch were 38.6 ± 0.7, 3.8 ± 0.3, and 41.1 ± 2.7 g kg-1, respectively. Before cultivating the red pepper, the hairy vetch plants were plowed in the same block. Hairy vetch plants nutrient content alone was not enough to fill the recommended fertilizer application for red pepper. So in addition to hairy vetch, a required amount of chemical fertilizer was added to fill the recommended amount. In particular, in the case of HV0.7N treatment, only half of the nitrogen chemical fertilizer as an additional fertilizer was applied compare to the HVN treatment. The total amount of N, P2O5, and K2O applied for HV0.7N and HVN treatment were 196.0, 90.0, and 141.0 and 280.0, 90.0, and 141.0 kg ha-1, respectively (Table 2).
Table 2. Fertilizer application amount (kg ha-1) using chemical fertilizer and hairy vetch under different fertilizer treatments.
Plant Cultivation and Maintenance Approximately 70 days old red pepper seedlings (C. annuum L. cv. Manita) were transplanted in three rows per block; each row contained ten seedlings. A total of 30 seedlings were planted per experimental block. The distance between the rows was 100 cm and the distance between two plants was 40 cm. The experimental blocks were equipped with drip irrigation system and the soil moisture content of the blocks was maintained at field capacity. The pepper plants were cultivated for another 163 days in the experimental blocks.
Plant Sampling and Nutrient Content Analysis After 163 days, five red pepper plants from each experimental blocks were randomly selected and harvested. The average value of the five plants were considered as one replication. Leaf, fruit, stem, and root samples were collected separately. The collected samples were dried in an oven at 70°C for three days and dry weights were measured. Macronutrient contents in the plant tissues were measured using method of soil and plant analysis (RDA, 2000). The total N contents in the plant tissues were measured using a Kjeldahl analyzer (Kjeltec 8400, FOSS analytical, Hillerød, Denmark). Plant P contents were measured by the ammonium metavanadate method. Plant K contents were measured using inductively coupled plasma optical emission spectrometry (ICP-OES) (SDS-720, GBC Scientific Equipments, Braeside, VIC, Australia). The plant nutrient use efficiencies were calculated using following equation:
| $$N_u=\frac{N_f-N_c}F\times100$$ | (Eq. 1) |
where is plant nutrient use efficiency (%), is plant nutrient uptake of treatments (kg ha-1), is plant nutrient uptake of control (kg ha-1), and is fertilizer applied in the soil (kg ha-1). The fertilizer application amount was set to P2O5 and K2O, however they were converted to P and K when calculating the nutrient use efficiency.
Rhizosphere Soil Sampling and Analysis of Soil Chemical Properties Five rhizosphere soil samples (within a 10 cm radius of the roots and a 15 cm depth; approximately 100 g) were randomly collected from each experimental block, pooled together, mixed, and dried after the red peppers were harvested. The dried soil samples were used for the analysis of soil chemical properties. Soil pH, electrical conductivity (EC), soil organic matter content (OM), and available P content (Av. P2O5) were analyzed using standard laboratory protocols. Soil nitrate and ammonia contents were analyzed using a Kjeldahl analyzer. The concentration of other nutrients, such as K, Ca, and Mg, were measured through ICP-OES.
Soil Enzyme Activity Three rhizosphere soil samples were collected twice from each experimental block 10 days after transplanting and harvesting. The soil samples were pooled together as one replication. The collected soil samples were immediately transported to the laboratory and soil enzymatic activity were measured within a week. Soil dehydrogenase activity was determined following the method using triphenyltetrazolium chloride dye (Casida et al., 1964). Soil β-glucosidase and phosphatase activities were determined colorimetrically as the concentration of p-nitrophenol produced after the incubation of 1 g of soil (37°C for 1 h) in 0.25 mL toluene, 4 mL of modified universal buffer (MUB; pH 6.5), and 0.025 M p-nitrophenyl-β-D-glucopyranoside or 0.115 M disodium p-nitrophenyl phosphate, respectively (Tabatabai and Bremner, 1969; Eivazi and Tabatabai, 1988). Soil urease activity was quantified colorimetrically as the NH4+ produced after incubating 5 g of soil in 2.5 mL of 0.08 M urea solution and 50 mL of 1 M potassium chloride solution at 37°C for 2 h (Kandeler and Gerber, 1988).
Statistical Analysis The average value of five plants from a single experimental block was considered as one replication. All measured data were evaluated using SPSS statistical package (IBM, New York, NY, USA). Data were analyzed with Duncan's new multiple range test (DMRT) after an analysis of variance (ANOVA) at the significance level of 0.05.
Results
CF, HV0.7N, and HVN treatments significantly increased total fresh and dry weight and fruit, leaf, and stem dry weight compared to the control (Table 3). In all treatments, root dry weight was not increased compared to the control. CF and HVN treatments significantly increased total, leaf, and stem dry weight compared to the HV0.7N treatment. No differences were observed between the CF and HVN treatments. Significantly higher macronutrient contents of the fruit tissue observed for CF, HV0.7N, and HVN treatments compared to control (Table 4). CF and HVN treatments showed significantly higher N and K contents of the total tissue than in the CON and HV0.7N treatment. All fertilizer treatments showed significantly higher N, P, and K uptake of the fruit tissue than in the control (Table 5). CF and HVN treatments significantly increased N, P, and K uptake of the leaf and total tissues compared to the CON and HV0.7N treatment. No differences of macronutrient uptake were observed in the root tissue. Even though different sources of N were applied, it did not have an effect on the N use efficiency by the red pepper (Table 6). However, P and K use efficiency in HV0.7N treatment were significantly lower than in the CF and HVN treatments.
Table 3. Total fresh weight and organ dry weight (kg ha-1) of red pepper plant under different fertilizer treatments.
†Each value represents the mean of 3 replications ± standard error.
§Each value in a column followed by the same letter are not significantly different by Duncan's multiple range test at p ≤ 0.05.
Table 4. Macronutrient (N, P, and K) content in red pepper plants under different fertilizer treatments.
†Each value represents the mean of 3 replications ± standard error.
§Each value in a column followed by the same letter are not significantly different by Duncan's multiple range test at p ≤ 0.05.
Table 5. Macronutrient (N, P, and K) uptake of red pepper plants under different fertilizer treatments.
†Each value represents the mean of 3 replications ± standard error.
§Each value in a column followed by the same letter are not significantly different by Duncan's multiple range test at p ≤ 0.05.
Table 6. Macronutrient (N, P, and K) use efficiency of red pepper plants under different fertilizer treatments.
| Treatments† | Nutrient use efficiency (%) | ||
| N | P | K | |
| CF | 62.6 ± 5.59†a§ | 21.8 ± 2.53ab | 163.4 ± 15.22a |
| HVHN | 49.8 ± 4.50a | 16.8 ± 2.52b | 68.1 ± 5.88b |
| HVN | 63.9 ± 4.02a | 25.6 ± 0.89a | 166.9 ± 11.25a |
†Each value represents the mean of 3 replications ± standard error.
§Each value in a column followed by the same letter are not significantly different by Duncan's multiple range test at p ≤ 0.05.
Analysis of soil chemical properties after experiment showed that the fertilizer treatments affected soil characteristics (Table 1). All fertilizer-applied experimental blocks had significantly higher soil EC and available P than the control blocks. Significantly higher available P was observed in the HVN treatment than CF and HV0.7N treatments. Hairy vetch-applied experimental blocks significantly increased pH and Ca content compared to the chemical fertilizer-applied blocks. Experimental blocks treated with hairy vetch had significantly higher soil organic matter content than blocks treated with chemical fertilizer alone.
Fertilizer applications significantly influenced soil microbial activities. No significant differences were observed in phosphatase activity between the treatments in 10 days after transplanting; however, CF treatments had significantly higher phosphatase activity than the control and the other treatments in 10 days after harvesting (Fig. 1A). Significantly higher dehydrogenase activity was observed in the hairy vetch-applied experimental blocks in 10 days after transplanting and harvesting (Fig. 1B). All fertilizer-applied experimental blocks had significantly higher urease activity than the control blocks in 10 days after transplanting; however, no significant differences were observed in urease activity between the treatments in 10 days after harvesting (Fig. 1C). Significantly higher β-glucosidase activity was observed in the hairy vetch-applied experimental blocks in 10 days after transplanting; however, HVN treatment had significantly higher β-glucosidase activity than the control and the other treatments in 10 days after harvesting (Fig. 1D).
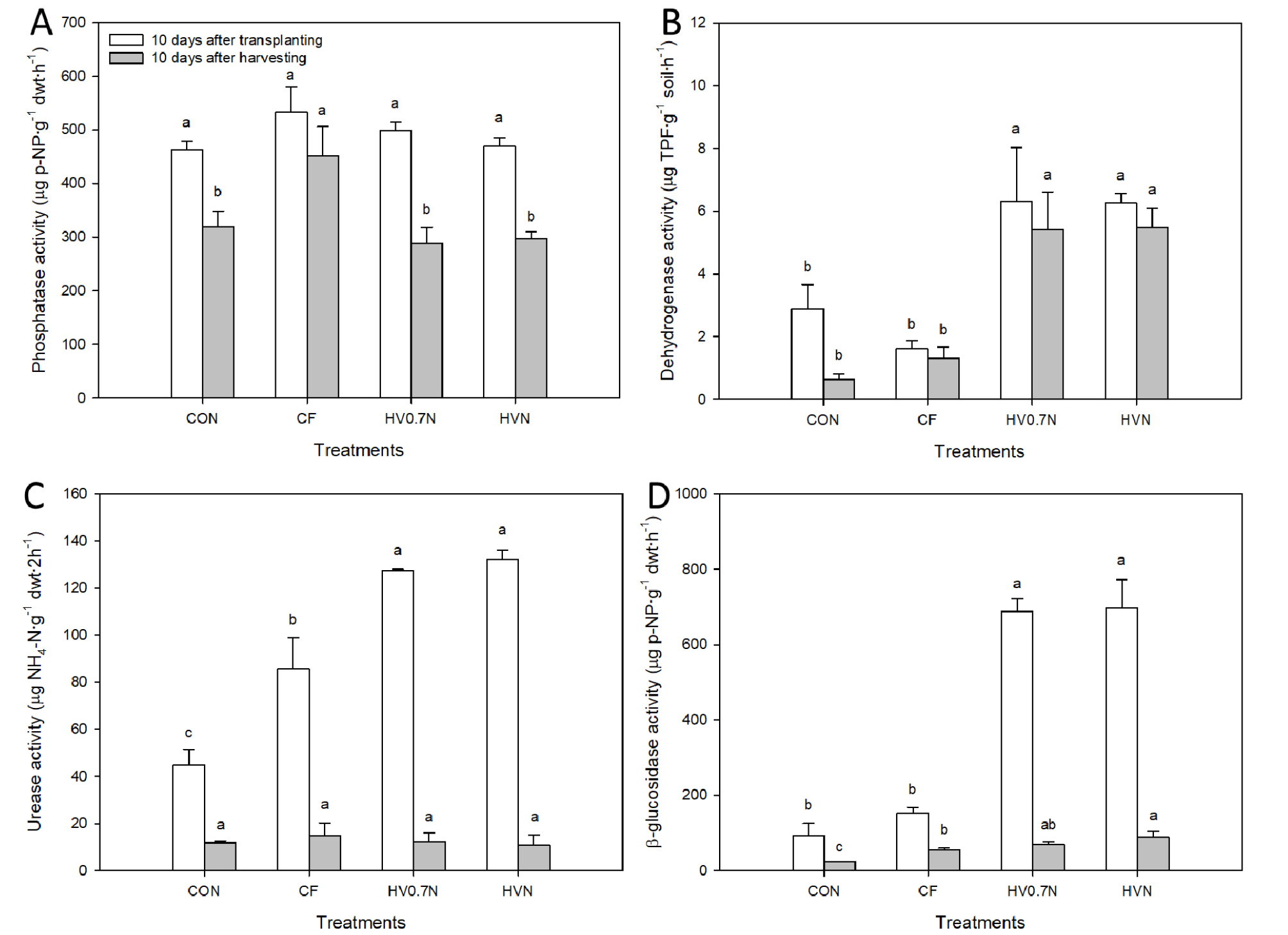
Fig. 1.
Soil enzyme activities of experimental blocks under different fertilizer treatments at 10 days after transplanting and harvesting red pepper plants. (A) phosphatase activity, (B) dehydrogenase activity, (C) urease activity, and (D) β-glucosidase activity. CON: control, CF: chemical fertilizer, HV0.7N: hairy vetch and 0.5N additional fertilizer, HVN: hairy vetch and N additional fertilizer. Bars with same letters are not statistically different according to Duncan’s multiple range test at p ≤ 0.05.
Discussion
To increase the growth and yield of facility-grown crops, nutrient management should be done properly. In general, the crop yield depends on the supply of nitrogen when there are enough other nutrients in the soil (Keeling et al., 2003; Hamnér et al., 2017). The CF, HV0.7N, and HVN treatments resulted in a significant increase in total fresh weight and fruit dry weight of red pepper (Table 3). Incorporating hairy vetch with chemical fertilizer treatments (HV0.7N and HVN) resulted in total fresh weight and fruit yield similar to the CF treatments. Only CF and HVN treatments promote largely the total, leaf, and stem dry weight of red pepper. However, the fruit dry weight is most important to analyze the effect of the fertilizer treatments. Previous studies reported that conventional chemical fertilizer and incorporating hairy vetch had a similar growth and yield promoting effect on tomato (Campiglia et al., 2010) and corn (Seo et al., 2000). However, the crop yield was decreased when the hairy vetch was only applied without chemical fertilizers, and when other green manure crops were used other than the hairy vetch (Sung et al., 2008; Suge et al., 2011). It is preferable to use green manure crop to reduce the usage of chemical fertilizer rather than to incorporate it alone. Therefore, determining the fertilizer dose through precise examination of the soil environment is important, when using green manure crops.
Through plant tissue analysis, the nutrient balance can be calculated, and furthermore, it can be determined how much nutrient of the soil has been used (Dang, 2005). CF, HV0.7N and HVN treatments showed that the N and K content of leaves and fruits and total crops increased compared to CON, but no trend was observed for P content in many organs (Table 4). However, because no significant difference in the macronutrients content in the CF, HV0.7N, and HVN treatments were observed in fruit, which is the final product of the facility cultivated red peppers, the quality of fruit in terms of nutrition is judged to be maintained. Furthermore, while P and K contents of red pepper fruits were maintained (Table 4), the P and K use efficiency of HV0.7N were lower than CF treatment (Table 6). However, there were no significant differences in P and K use efficiency between HVN and HV0.7N. This result indicates that the macronutrient content of the red pepper fruit incorporating with hairy vetch appears similar levels to chemical fertilizers, consistent with those found in previous studies (Muchanga et al., 2017; Selvakumar et al., 2018b). In this study, even though N application was reduced with incorporating hairy vetch, N uptake of fruit and N use efficiency of red peppers were no significant different compared to chemical fertilizer treatment group (Tables 5 and 6). This results confirm that incorporating hairy vetch can reduce the use of chemical N fertilizer while ensuring productivity of red peppers.
Dehydrogenase, urease, β-glucosidase, and phosphatase are involved in biological oxidation, N cycle, C cycle, and P cycle, respectively (Subhani et al., 2001; Adetunji et al., 2017). Higher dehydrogenase, urease, and β-glucosidase activities were observed in the hairy vetch applied soils (HV0.7N and HVN) after red pepper transplanting suggest that incorporating hairy vetch promote the biological oxidation and N and C cycle in the soil (Fig. 1B, C, and D). However, when after red pepper cultivation, only the dehydrogenase activity was maintained, and the urease and β-glucosidase activity was decreased to less than one tenth in hairy vetch applied soils. Although β-glucosidase activities were higher than CON or CF treatment, their absolute activity levels were lower than that in the red pepper transplanting season. Therefore, in early stage of red pepper cultivation, incorporating hairy vetch can promote the biological oxidation and N and C cycles through the soil microorganisms, which will help in the facility production of the red peppers (Selvakumar et al., 2018a). Applying green manure crops, including hairy vetch, to the soil increases the microbial biomass in the soil and their enzyme activity (Piotrowska and Wilczewski, 2012). In particular, incorporating hairy vetch increases the substrate of β-glucosidase, thus increasing β-glucosidase activity, and there is positive correlation between the degradation of green manure crop material and the β-glucosidase activity (Stott et al., 2010). There were no significant differences in the P content of the red pepper in Table 4, and the phosphatase activity in Fig. 1A, known to be involved in the soil P cycle, did not vary depending on incorporating hairy vetch. To increase crop yield through the soil management in the facility, the importance of rhizosphere soil management, including microorganisms, was identified.
Conclusion
Three different fertilizer-applied treatments for facility-grown red pepper (Capsicum annuum L.) were evaluated in this study. Our experimental results show that even though chemical N fertilizer application was reduced with incorporating hairy vetch (Vicia villosa Roth), N uptake of fruit and N use efficiency of red peppers were no significant different compared to chemical fertilizer only treatment. Different from chemical fertilizer, incorporating hairy vetch residue make soil chemical properties better by increasing the pH, EC, soil organic matter, and available P. Moreover, incorporating hairy vetch increased soil microbial activities such as dehydrogenase, urease, and β-glucosidase. These results suggested that incorporating hairy vetch as green manure crop can be as helpful at increasing red pepper yield and reducing chemical fertilizers. Further research for these organic fertilizer application on facility-grown crops over a long period of time can provide more efficient and productive method on soil management to reduce chemical fertilizer and to improve soil microbial activity in crop cultivating facility.





